Get the WHOLE Story About CNS MR Contrast
The phase IV, double-blind, multi-center, randomized crossover study reveals there’s more to the TRUTH when comparing the clinical benefits of ProHance® (gadoteridol) and Gadavist® (gadobutrol):
- ProHance and Gadavist produce no diagnostic differences.1, 2
- The 2-fold higher concentration of Gadavist provides no benefits for routine morphologic imaging.1, 2
- Gadavist does not demonstrate the clinical benefits for diagnostic performance associated with high relaxivity in CNS MRI.1-3
- That means only MultiHance® (gadobenate dimeglumine) offers the highest relaxivity in CNS MRI to improve visualization of brain lesions.3-7
Case Studies
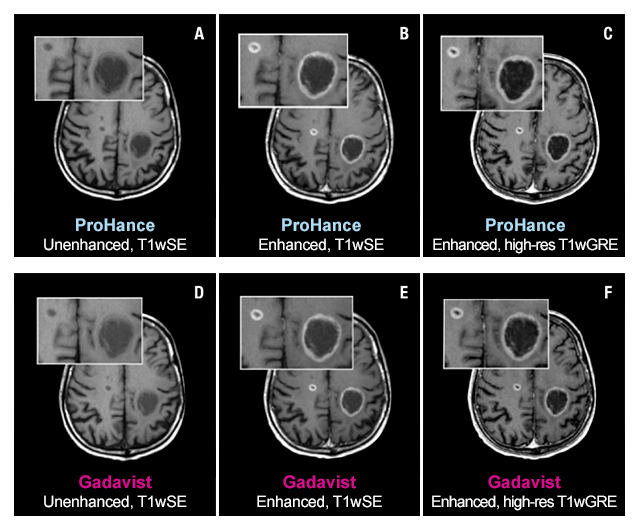
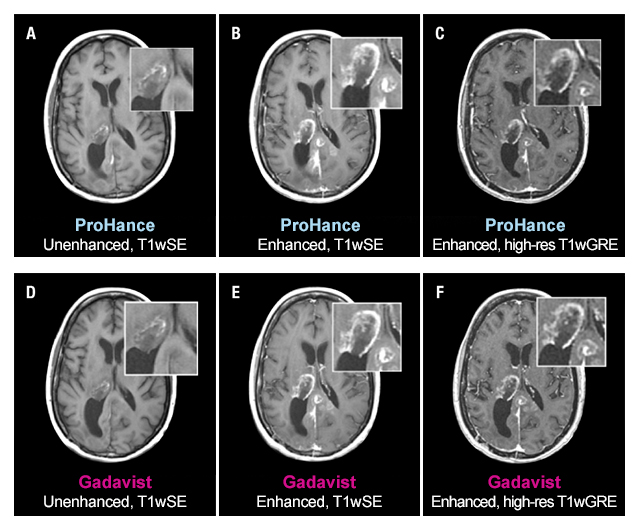
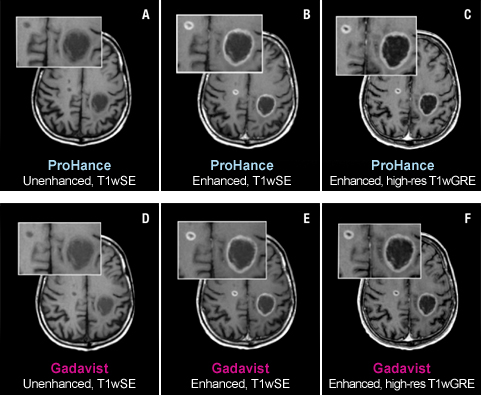
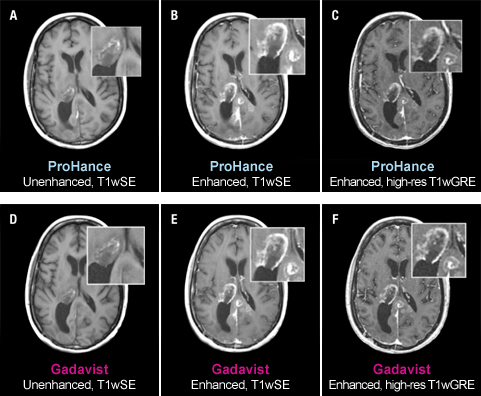
The TRUTH study results demonstrate no differences in contrast enhancement of lesion morphology.1
Case Study 1
Case Study 2
61-year-old male with brain metastases from primary lung cancer. Two lesions clearly seen in both exams show no differences in contrast enhancement or in the morphology of lesions.1
51-year-old female with glioblastoma multiforme. Rim-enhancing mass in right thalamus with extension into the posterior interhemispheric region is clearly seen in both examinations. No differences in contrast enhancement or in the morphology of lesions are apparent.1
These are representational images from reference studies. Individual results may vary. REFERENCE: 1. Maravilla K, Smith M, Vymazal J, et al. Are there differences between macrocyclic gadolinium contrast agents for brain tumor imaging? Results of a multicenter intraindividual crossover comparison of gadobutrol with gadoteridol (The TRUTH study). AJNR Am J Neuroradiol. 2015;1:14-23, doi: 10.3174/ajnr.A4154.
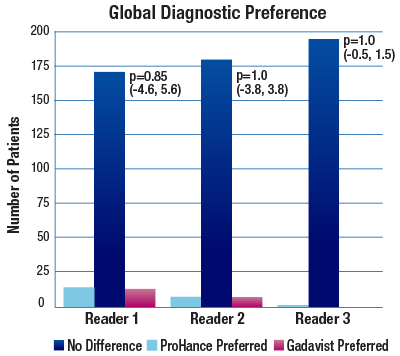
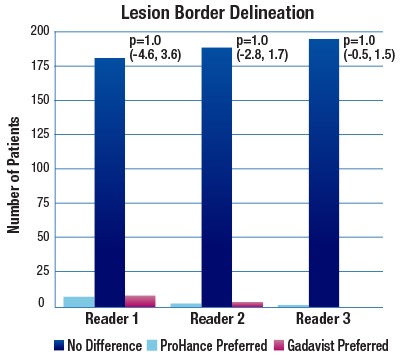
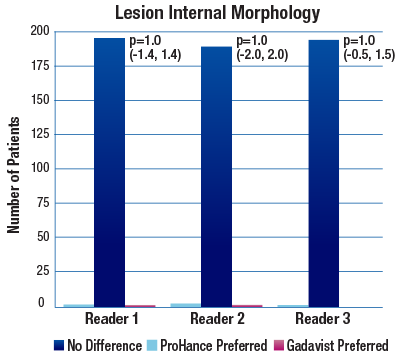
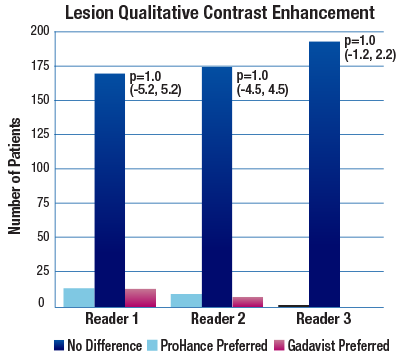
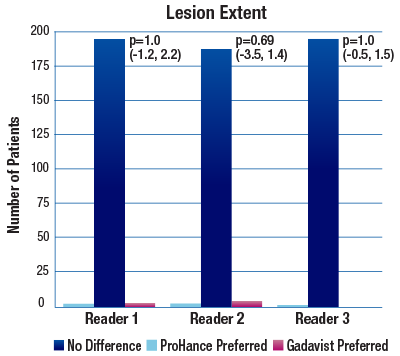
The 95% confidence intervals for all qualitative assessments confirm that ProHance is not inferior to Gadavist.1
No significant differences noted by any reader for any parameter:1
- Global Diagnostic Preference
- Lesion Border Delineation
- Lesion Internal Morphology
- Lesion Qualitative Contrast Enhancement
- Lesion Extent
The TRUTH Speaks for Itself
Discover the advantage that ProHance and Bracco’s suite of MR products can bring to your practice.
Visit our website or contact your rep today.
REFERENCES: 1. Maravilla K, Smith M, Vymazal J, et al. Are there differences between macrocyclic gadolinium contrast agents for brain tumor imaging? Results of a multicenter intraindividual crossover comparison of gadobutrol with gadoteridol (The TRUTH study). AJNR Am J Neuroradiol. 2015;1:14-23, doi: 10.3174/ajnr.A4154. 2. Gadavist (gadobutrol) injection, full Prescribing Information. Whippany, NJ: Bayer HealthCare Pharmaceuticals Inc.; December 2014. 3. Seidl Z, Vymazal J, Mechl M, et al. Does higher gadolinium concentration play a role in the morphologic assessment of brain tumors? Results of a multicenter intraindividual crossover comparison of gadobutrol versus gadobenate dimeglumine (the MERIT study). AJNR. 2012;33(6):1050-1058. 4. Rohrer M, Bauer H, Mintorovitch J, et al. Comparison of magnetic properties of MRI contrast media solutions at different magnetic field strengths. Invest Radiol. 2005;40:715-724. 5. Pintaske J, Martirosian P, Graf H, et al. Relaxivity of gadopentetate dimeglumine (Magnevist), gadobutrol (Gadovist), and gadobenate dimeglumine (MultiHance) in human blood plasma at 0.2, 1.5 and 3 Tesla. Erratum Invest Radiol. 2006;41:213-221. 6. Rowley HA, Scialfa G, Gao RY, et al. Contrast-enhanced MR imaging of brain lesions: a large-scale intraindividual crossover comparison of gadobenate dimeglumine versus gadodiamide. AJNR. 2008;29:1684-1691. 7. Maravilla KR, Maldijan JA, Schmalfuss IM, et al. Contrast enhancement of central nervous system lesions: multicenter intraindividual crossover comparative study of two MR contrast agents. Radiology. 2006;240(2):389-400.
INDICATIONS AND USAGE FOR PROHANCE
Central Nervous System
ProHance (Gadoteridol) Injection is indicated for use in MRI in adults and children over 2 years of age to visualize lesions with abnormal vascularity in the brain (intracranial lesions), spine, and associated tissues.
Extracranial/Extraspinal Tissues
ProHance is indicated for use in MRI in adults to visualize lesions in the head and neck.
IMPORTANT SAFETY INFORMATION
INDICATIONS AND USAGE FOR PROHANCE
Central Nervous System
ProHance (Gadoteridol) Injection is indicated for use in MRI in adults and children over 2 years of age to visualize lesions with abnormal vascularity in the brain (intracranial lesions), spine, and associated tissues.
Extracranial/Extraspinal Tissues
ProHance is indicated for use in MRI in adults to visualize lesions in the head and neck.
IMPORTANT SAFETY INFORMATION
WARNING: NEPHROGENIC SYSTEMIC FIBROSIS
Gadolinium-based contrast agents (GBCAs) increase the risk for NSF among patients with impaired elimination of the drugs. Avoid use of GBCAs in these patients unless the diagnostic information is essential and not available with non-contrasted MRI or other modalities. NSF may result in fatal or debilitating systemic fibrosis affecting the skin, muscle and internal organs.
- The risk for NSF appears highest among patients with:
- Chronic, severe kidney disease (GFR <30 mL/min/1.73m2), or
- Acute kidney injury.
- Screen patients for acute kidney injury and other conditions that may reduce renal function. For patients at risk for chronically reduced renal function (e.g. age > 60 years, hypertension or diabetes), estimate the glomerular filtration rate (GFR) through laboratory testing.
- For patients at highest risk for NSF, do not exceed the recommended ProHance dose and allow a sufficient period of time for elimination of the drug from the body prior to re-administration [See WARNINGS].
As with all paramagnetic agents, caution should be exercised in patients with deoxygenated sickle erythrocytes and renal insufficiency with or without hepatic imapairment. The possibility of a reaction, including serious, life threatening, or fatal, anaphylactic or cardiovascular reactions, or other idiosyncratic reactions, should always be considered, especially in those patients with a history of a known clinical hypersensitivity or a history of asthma or other allergic disorders.
Please consult full Prescribing Information for ProHance including boxed WARNING contained within this website.
INDICATIONS AND USAGE FOR MULTIHANCE
MultiHance is a gadolinium-based contrast agent indicated for use in:
- Magnetic resonance imaging (MRI) of the central nervous system (CNS) in adults and children over 2 years of age to visualize lesions with abnormal blood-brain barrier or abnormal vascularity of the brain, spine, and associated tissues and
- Magnetic resonance angiography (MRA) to evaluate adults with known or suspected renal or aorto-ilio-femoral occlusive vascular disease.
IMPORTANT SAFETY INFORMATION
WARNING: NEPHROGENIC SYSTEMIC FIBROSIS
Gadolinium-based contrast agents (GBCAs) increase the risk for NSF among patients with impaired elimination of the drugs. Avoid use of GBCAs in these patients unless the diagnostic information is essential and not available with non-contrasted MRI or other modalities. NSF may result in fatal or debilitating systemic fibrosis affecting the skin, muscle and internal organs.
- The risk for NSF appears highest among patients with:
- Chronic, severe kidney disease (GFR <30 mL/min/1.73m2), or
- Acute kidney injury.
- Screen patients for acute kidney injury and other conditions that may reduce renal function. For patients at risk for chronically reduced renal function (e.g. age > 60 years, hypertension or diabetes), estimate the glomerular filtration rate (GFR) through laboratory testing.
- For patients at highest risk for NSF, do not exceed the recommended MultiHance dose and allow a sufficient period of time for elimination of the drug from the body prior to re-administration. [See Warnings and Precautions (5.1)]†.
Anaphylactic and anaphylactoid reactions have been reported, involving cardiovascular, respiratory, and/or cutaneous manifestations ranging from mild to severe. The possibility of a reaction should always be considered, especially in those patients with a history of a known clinical hypersensitivity or a history of asthma or other allergic disorders.
†Please consult full Prescribing Information for MultiHance including boxed WARNING contained within this website.
You are encouraged to report negative side effects of prescription drugs to the FDA.
Visit www.fda.gov/medwatch or call 1-800-FDA-1088.
ProHance® is manufactured for Bracco Diagnostics Inc. by BIPSO GmbH — 78224 Singen (Germany).
MultiHance® is manufactured for Bracco Diagnostics Inc. by BIPSO GmbH — 78224 Singen (Germany) and by Patheon Italia SpA, Ferentino, Italy.
All trademarks and registered trademarks are the property of their respective owners.
Copyright © 2015 Bracco Diagnostics Inc.
Privacy Policy
|
Terms of Use|
Imprint|
THIS SITE IS INTENDED FOR U.S. AUDIENCES ONLY